Digital transformation in the pharmaceutical industry
Let us guide you
Fabrity is a software house specializing in bespoke software development, product design, cloud transformation, and low-code solutions. For our clients, we build software development teams covering all skills and project roles. We can help you augment your IT staff to meet the growing demands of your business or build a dedicated team for a complex project.
Who we work for
The top global pharmaceutical brands have chosen to realize complex long-term software projects with us. In fact, 90% of our clients have been working with us for more than five years.







Quality management systems (QMS)
Quality monitoring systems
Product lifecycle management
Compliance with pharmaceutical regulations
Compliance with financial regulations
Compliance with internal regulations
Handling of pharmaceutical product complaints
Operations automation
Master data management
Quality management systems (QMS)
Building a document management system to ensure compliance with GMP standards in a pharmaceutical factory
Client
A leading OTC manufacturer
Business goal
Ensure compliance with Good Manufacturing Practices (GMP) by providing up-to-date procedures to every employee involved in the production process.
Technology stack
- K2 blackpearl
- .NET, Angular
- MS SQL Server
- SharePoint
- Office Web Apps
Business outcome
- Ensured GMP compliance: the quality control department can easily ensure and monitor compliance of the workplace instructions with good manufacturing practices.
- Ensured high quality: every employee always has up-to-date workplace instructions.
- Streamlined the process: the document management process is now highly automated.
- Reduced error risk: advanced mechanisms ensure that documents are correct and have been reviewed and accepted.
How we helped
- Built standard and custom document management workflows.
- Built the digital document repository and archive.
- Automated the process of creating, reviewing, and accepting documents related to GMP standards in the pharmaceutical factory.
- Created an audit feature making it possible to check the correctness of created documents.
- Created a testing feature to check if employees know the most up-to-date documents and instructions.
- Built a feature making it possible to track down all paper copies of the documents.
- Added a feature allowing for group work on documents.
- Integrated with Office Web Apps and Active Directory.
- Provided maintenance and further development.
Client
A leading OTC manufacturer
Business goal
Ensure compliance with GMP standards in a pharmaceutical factory through proper management of deviations, customer complaints, and internal audits
Technology stack
- Hangfire
- .NET
- Angular
- PostgreSQL
- MS Azure
Business outcome
- Ensured quality: all deviations from product standards are carefully tracked and handled to prevent more serious quality issues.
- Quality monitored: internal audits make it possible to detect problems and take appropriate corrective actions.
- Ensured GMP standards are met: the system monitors compliance with good manufacturing practices in the pharmaceutical factory.
How we helped
- Prepared a business analysis.
- Prepared a functional specification of the solution.
- Developed the solution.
- Tested the solution internally and conducted an acceptance test with the client.
- Deployed the solution in the cloud using MS Azure.
- Prepared instructions and trained users.
- Prepared validation documentation according to GMP requirements.
Building a new product development system for a leading OTC manufacturer
Client
A leading drug manufacturerBusiness goal
Streamline the process of developing a new product from idea through formulation acceptance, designing and printing packages, to full registration by the state authoritiesTechnology stack
- K2 blackpearl
- .NET
- Entity Framework
- MS SQL Server
- SSIS
- SSAS
- SSRS
- SharePoint
- AngularJS
- JavaScript
Business outcome
- Streamlined the process: the system ensures that all stages are fulfilled, all stakeholders and decision makers are involved, and all the required documents are attached.
- Shortened time-to-market: a new or modified pharmaceutical product is registered and released to the market faster.
- Enhanced monitoring: all elements related to development of a new product (specification, documentation, project team, decision makers, registration procedure, packaging, etc.) are handled by one system.
How we helped
- Prepared a thorough business analysis describing the concept of the entire system: its features, forms, views, business logic, and permissions.
- Designed and built functional and technical aspects of the system.
- Designed and built the UI.
- Designed and built the system.
- Implemented the system on the client’s premises.
- Trained the client’s employees.
- Prepared and handed over the system documentation.
- Worked in iterations according to Agile methodology.
- Provided maintenance services.
Client
A multinational pharmaceutical companyBusiness goal
Ensure compliance with state regulations requiring that pharmaceutical companies report their trade in medical products to the central system (ZSMOPL)Technology stack
- Hangfire
- .NET
- Angular
- Windows Communication Foundation (WCF)
- MS SQL Server
Business outcome
- Ensured compliance with state regulations: reports on trade in medical products are sent automatically each day.
- Reduced manual labor: the system can work automatically when it comes to data validation and sending.
- Ensured legal requirements are met over time: adjusts the data format to the changing requirements of the central system (ZSMOPL).
How we helped
- Designed and built a custom solution to meet all of the client’s requirements.
- Built an automated mechanism to validate and process data coming from different sources (SAP, Excel files).
- Added a feature making it possible to edit data manually in case of discrepancies.
- Built a feature making it possible to send reports to the central system (ZSMOPL) both automatically and after a manual data verification.
- Ensured data sent conform with the data format required by the central monitoring system (ZSMOPL), which changes over time.
- Added email notifications for business users informing them that data were sent successfully or returning an error log in case of failure.
- Built a user-friendly UI.
- Provided maintenance services.
Client
A multinational pharmaceutical and healthcare companyBusiness goal
Ensure compliance with US financial regulationsTechnology stack
- K2 blackpearl
- .NET
- MS SQL Server
Business outcome
- Ensured compliance: all business processes are compliant with US financial regulations.
- Streamlined business processes: low-code apps are easy to learn, intuitive, and highly reliable.
- Ensured security: remote users use a VPN tunnel to access the company’s Intranet.
How we helped
- Designed and built over 100 business processes based on a low-code platform.
- Optimized workflows for field-force users working remotely.
- Integrated the solution with the company’s SAP and Workday systems.
- Prepared the solution documentation and trained users.
- Provided technical support, maintenance, and further development.
Compliance with internal regulations
Client
A multinational pharmaceutical and biotechnology company
Business goal
Ensure both compliance of marketing activities with internal regulations and their cost control
Technology stack
- K2 workflow engine
- Hangfire
- .NET
- MS SQL Server
- Angular
Business outcome
- Improved work efficiency: time-consuming tasks were sped up and the amount of manual labor was considerably reduced.
- Ensured internal compliance: the budget of marketing activities is closely monitored to detect any non-compliance.
- Ensured better control over processes: users can generate reports that make it possible to manage processes better.
How we helped
- Built a system to handle marketing activities.
- Identified 16 business processes of varying complexity to create acceptance paths for daily tasks.
- Acceptance paths were assigned to departments and managers to ensure flexibility and that the system would work regardless of employee rotation.
- Built both functional and technical aspects of the system.
- Implemented the final solution.
- Integrated the solution with other systems in the organization (e.g., an HR system).
- Trained the client’s employees.
- Prepared and handed over the solution’s documentation.
- Provided further development and maintenance.
Handling of pharmaceutical product complaints
Client
A multinational pharmaceutical and healthcare companyBusiness goal
Ensure complaints related to pharmaceutical products coming from end clients (pharmacies, pharmaceutical wholesalers) are handled in compliance with legal regulations.Technology stack
- K2 Blackpearl
- ASP.NET
- Web Forms
- MS SQL Server
Business outcome
- Ensured compliance with the pharmaceutical regulations: complaint management process is regularly audited by the Polish Chief Pharmaceutical Inspectorate and all audits are positive.
- Ensured compliance with GAMP 5 regulations: the system achieves the desired, positive results in an accurate, consistent, and repeatable manner, which was documented during the validation process.
- Streamlined the process: complaints are handled fast, with due diligence, and while keeping in touch with end clients (pharmacies, pharmaceutical wholesalers).
- Met critical SLA requirements: technical support is provided quickly to solve any potential issues as soon as possible.
How we helped
- Organized a workshop with the client.
- Prepared a detailed process map.
- Designed the system.
- Developed the system.
- Tested the system and deployed it on the client’s premises.
- Integrated the system with the client’s SAP to ensure smooth data flow.
- Integrated the system with Kamsoft software used by pharmacies in Poland allowing them to send complaints directly.
- Ensured the system is compliant with GAMP 5 requirements related to the validation of IT systems working in the area of pharmaceutical manufacturing.
- Supported the client during inspections performed by the Polish Chief Pharmaceutical Inspectorate.
- Provided technical support and further development to ensure the system is compliant with legal regulations.
Operations automation
Client
A leading OTC manufacturerBusiness goal
Streamline the financial document management processTechnology stack
- K2 blackpearl
- .NET
- Angular
- MS SQL Server
Business outcome
- Reduced manual labor: the document management process has been streamlined and some financial settlements have been automated.
- Sped up the process: the system tracks progress and sends notifications to responsible persons if any action needs to be completed.
- Enhanced monitoring: the system allows you to create reports and make sure no step in the process or document is missing.
How we helped
- Prepared the business analysis and system specifications.
- Developed system back- and frontends.
- Performed internal tests.
- Performed acceptance tests for the client.
- Deployed the system.
- Provided maintenance and further development.
Master data management
Client
A leading OTC manufacturerBusiness goal
Ensure accurate business reporting by identifying correct dataTechnology stack
- .NET
- Angular
- MS SQL Server
- MS Azure
Business outcome
- Data quality ensured: the system makes it possible to identify golden records, i.e., the correct data variants.
- Accurate reporting ensured: reports based on correct data are trustworthy.
How we helped
- Performed a business analysis.
- Prepared the specifications of the solution.
- Developed the solution.
- Tested the solution (internal and acceptance tests).
- Deployed the solution in the cloud.
- Provided maintenance and further development.
Our services
Software engineering
For our enterprise clients, we build bespoke software solutions covering both core aspects of their business (new product development, quality management, compliance with legal regulations and industry standards), as well as daily operations of legal, finance, marketing, and HR departments.
Product design
With us you can take your product from idea to reality. We offer comprehensive UX consulting services starting from UX research, through defining, ideation (UX/UI design), to web and mobile development. We also provide support and maintenance services.
Low-code
We navigate pharmaceutical companies through their digital transformation journey allowing them to automate their business processes, reduce costs and manual labor, as well as ensure compliance with industry and law regulations. Low-code solutions, digital process automation (DPA), and robotic process automation (RPA)—all of these are parts of our automation and hyperautomation toolset.
Cloud
We help companies in their cloud transformation, advising on best strategy and solutions. We design cloud infrastructure, plan and execute cloud migration, build cloud-based software, and provide cloud managed services.
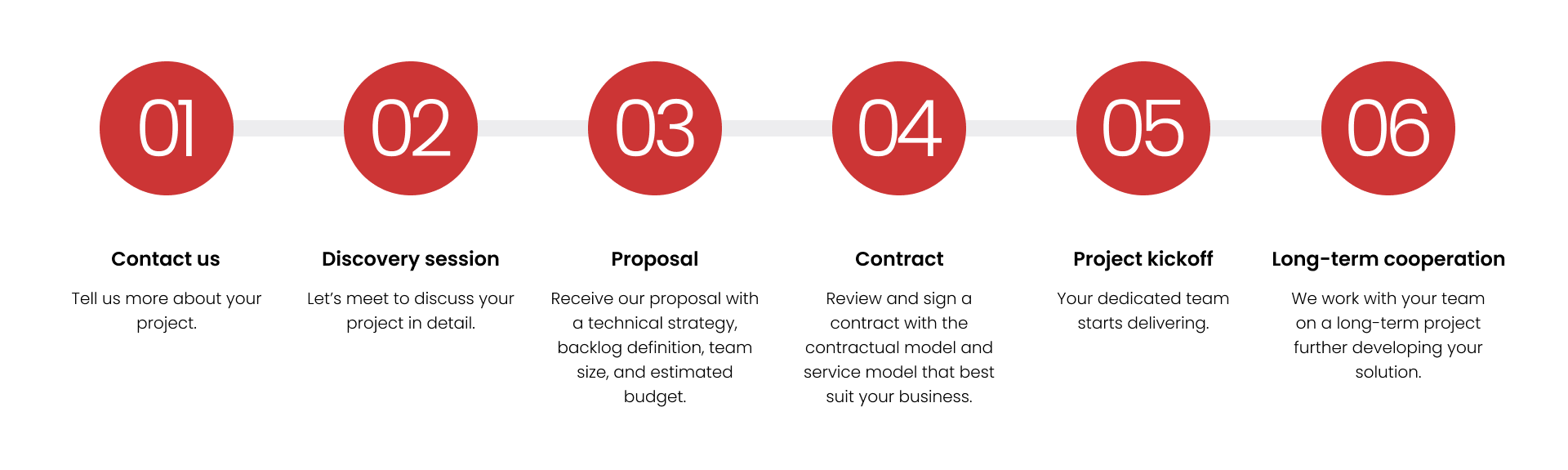
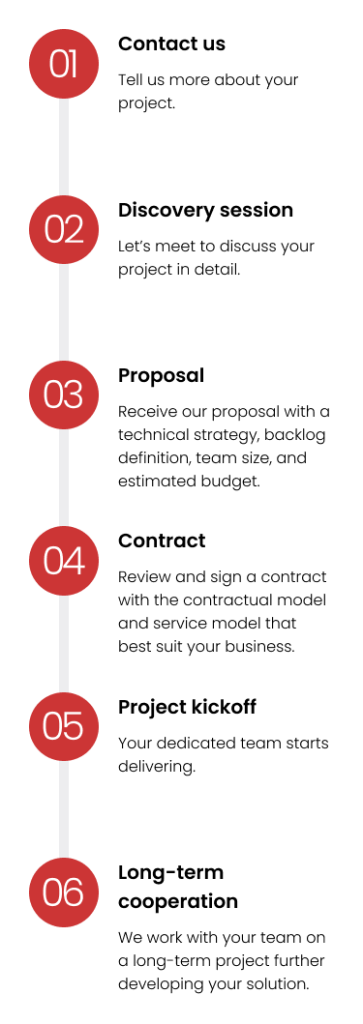
Build cross-functional teams with all roles and skills covered in under three months.
Choose the contractual model and service model that best suit your business.
Pay only for the software functionalities delivered.
Get advice on team composition, size, and seniority level; solution architecture; and project backlog.
Cover all risks: underperformance, absences, security, confidentiality, and GDPR requirements.
Ensure the best working conditions for your team: project onboarding, upskilling, best practices, and boosting motivation and engagement.
Work with a partner that provides hardware, software licenses, and office space (if needed).
Service models
A dedicated team managed by Fabrity
- Build a cross-functional team with all roles and skills covered.
- Benefit from our tools, processes, best practices, and infrastructure.
- Use a quoted time and materials (QT&M) model based on the volume of software delivered.
A team managed by the client
- Scale up your project team with our IT experts according to your current needs.
- We make sure your developers have everything to start working immediately (hardware, software licenses, ensured security, and GDPR requirements).
- Use a time and materials contract (T&M) with contracted flexibility.
A hybrid team
- Mix our experts with your team to strengthen it with the skills you need.
- Benefit from our software development best practices and processes.
- Use either a quoted time and materials (QT&M) or time and materials (T&M) model depending on how leadership roles are split between your team and our experts.
Software development hubs
- Set up your virtual software development hub quickly.
- Access the tech talent pool available in the CEE region.
- Scale up and down without incurring upfront costs.
How we work
Technology stack
We build project teams with software engineers who have expertise in leading or niche technologies.
Enterprise software development:






Web software development:





Mobile software development:




Data engineering:





Interested in working with a partner with experience in the pharmaceutical industry?
Drop us a line to see how we can help.
Book a free 15-minute discovery call
Let’s talk to see how we can help.



